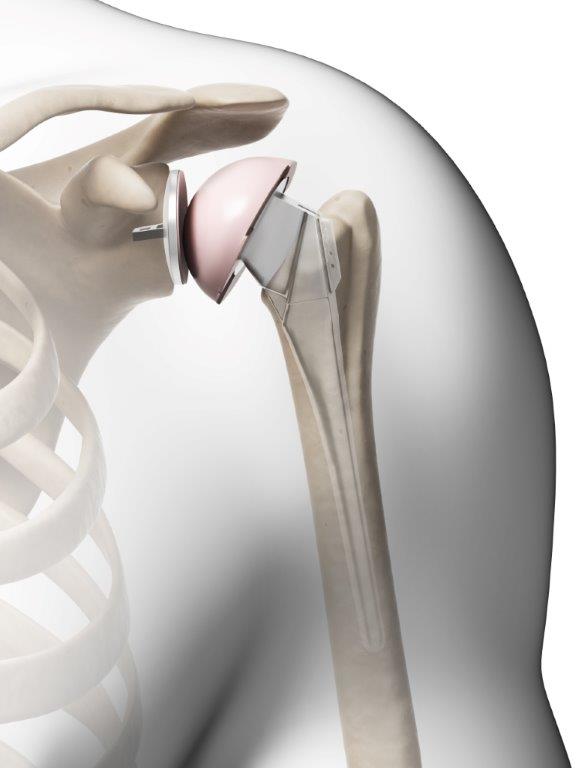
The U.S. Food and Drug Administration (FDA) recognizes that a Class I recall has been issued on the Zimmer Biomet Comprehensive Reverse Shoulder with a product description of Comprehensive Reverse Shoulder System Humeral Tray Model 115340 distributed between October of 2008 and September of 2015. The shoulder replacement systems are reported to have high fracture rates after being implanted for patients with rotator cuff tears with severe shoulder arthropathy. The fractures that can occur with the product can cause the need for revision surgeries, permanent loss of shoulder function, infection, or even death in rare instances. For contacts about the risks of your shoulder replacement system, consumers and medical providers can call (574)371-3071 or e-mail [email protected]. Problems can be reported to the FDA at https://www.accessdata.fda.gov/scripts/medwatch/index.cfm.
If you or a loved one has been injured by a recalled Zimmer Biomet product, or any medical device, drug, or other product, consult your medical provider and please contact Inserra Kelley Sewell, Personal Injury Attorneys to discuss your possible claim and entitlement to compensation for your damages.





